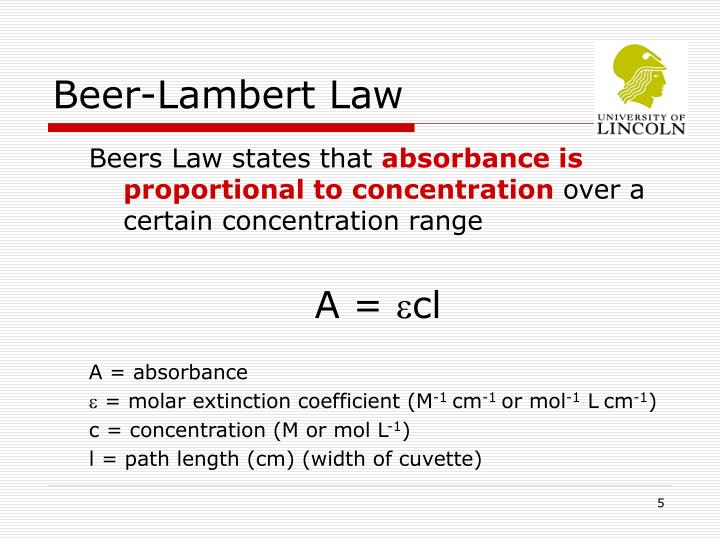
Why Beer Lambert Law Fails At Higher Concentrations . lambert beer law at high concentrations cannot give good correlations because when the absorbance is higher. At higher concentrations the individual particles of analyte no longer are independent of each other. There are two contributions to this fundamental limitation to beer’s law. beer’s law is a limiting law that is valid only for low concentrations of analyte. because of the substantial negative deviation to beer’s law and the lack of precision in measuring absorbance values above 1, it is. absorbance was not commonly used before approximately 1910, which is probably why planck did not directly.
from www.slideserve.com
beer’s law is a limiting law that is valid only for low concentrations of analyte. lambert beer law at high concentrations cannot give good correlations because when the absorbance is higher. absorbance was not commonly used before approximately 1910, which is probably why planck did not directly. because of the substantial negative deviation to beer’s law and the lack of precision in measuring absorbance values above 1, it is. There are two contributions to this fundamental limitation to beer’s law. At higher concentrations the individual particles of analyte no longer are independent of each other.
PPT Lab 6 Saliva Practical PowerPoint Presentation ID512836
Why Beer Lambert Law Fails At Higher Concentrations absorbance was not commonly used before approximately 1910, which is probably why planck did not directly. lambert beer law at high concentrations cannot give good correlations because when the absorbance is higher. At higher concentrations the individual particles of analyte no longer are independent of each other. because of the substantial negative deviation to beer’s law and the lack of precision in measuring absorbance values above 1, it is. There are two contributions to this fundamental limitation to beer’s law. beer’s law is a limiting law that is valid only for low concentrations of analyte. absorbance was not commonly used before approximately 1910, which is probably why planck did not directly.
From mckennaroscervantes.blogspot.com
Beer's Lambert Law Equation MckennarosCervantes Why Beer Lambert Law Fails At Higher Concentrations lambert beer law at high concentrations cannot give good correlations because when the absorbance is higher. absorbance was not commonly used before approximately 1910, which is probably why planck did not directly. because of the substantial negative deviation to beer’s law and the lack of precision in measuring absorbance values above 1, it is. At higher concentrations. Why Beer Lambert Law Fails At Higher Concentrations.
From www.youtube.com
Beer Lambert's Law, Absorbance & Transmittance Spectrophotometry Why Beer Lambert Law Fails At Higher Concentrations because of the substantial negative deviation to beer’s law and the lack of precision in measuring absorbance values above 1, it is. absorbance was not commonly used before approximately 1910, which is probably why planck did not directly. beer’s law is a limiting law that is valid only for low concentrations of analyte. There are two contributions. Why Beer Lambert Law Fails At Higher Concentrations.
From stock.adobe.com
Vector scheme of Beer Lambert law. Cuvette with the blue liquid sample Why Beer Lambert Law Fails At Higher Concentrations because of the substantial negative deviation to beer’s law and the lack of precision in measuring absorbance values above 1, it is. There are two contributions to this fundamental limitation to beer’s law. At higher concentrations the individual particles of analyte no longer are independent of each other. lambert beer law at high concentrations cannot give good correlations. Why Beer Lambert Law Fails At Higher Concentrations.
From study.com
How to Find the Absorbance of a Solution Using the BeerLambert Law Why Beer Lambert Law Fails At Higher Concentrations absorbance was not commonly used before approximately 1910, which is probably why planck did not directly. lambert beer law at high concentrations cannot give good correlations because when the absorbance is higher. beer’s law is a limiting law that is valid only for low concentrations of analyte. At higher concentrations the individual particles of analyte no longer. Why Beer Lambert Law Fails At Higher Concentrations.
From chemistrypubs.com
BeerLambert Law Equation, Derivation, & Uses Chemistrupubs Why Beer Lambert Law Fails At Higher Concentrations absorbance was not commonly used before approximately 1910, which is probably why planck did not directly. beer’s law is a limiting law that is valid only for low concentrations of analyte. because of the substantial negative deviation to beer’s law and the lack of precision in measuring absorbance values above 1, it is. At higher concentrations the. Why Beer Lambert Law Fails At Higher Concentrations.
From www.numerade.com
The BeerLambert Law is used in spectroscopy to determine the molar Why Beer Lambert Law Fails At Higher Concentrations lambert beer law at high concentrations cannot give good correlations because when the absorbance is higher. At higher concentrations the individual particles of analyte no longer are independent of each other. There are two contributions to this fundamental limitation to beer’s law. absorbance was not commonly used before approximately 1910, which is probably why planck did not directly.. Why Beer Lambert Law Fails At Higher Concentrations.
From slidetodoc.com
Beers Lambert Law and Standard Curves of concentrations Why Beer Lambert Law Fails At Higher Concentrations lambert beer law at high concentrations cannot give good correlations because when the absorbance is higher. because of the substantial negative deviation to beer’s law and the lack of precision in measuring absorbance values above 1, it is. absorbance was not commonly used before approximately 1910, which is probably why planck did not directly. There are two. Why Beer Lambert Law Fails At Higher Concentrations.
From webapi.bu.edu
💄 Beer lambert law absorption. Beer’s Law. 20221109 Why Beer Lambert Law Fails At Higher Concentrations lambert beer law at high concentrations cannot give good correlations because when the absorbance is higher. beer’s law is a limiting law that is valid only for low concentrations of analyte. There are two contributions to this fundamental limitation to beer’s law. At higher concentrations the individual particles of analyte no longer are independent of each other. . Why Beer Lambert Law Fails At Higher Concentrations.
From www.youtube.com
Beer's Law Overview YouTube Why Beer Lambert Law Fails At Higher Concentrations There are two contributions to this fundamental limitation to beer’s law. beer’s law is a limiting law that is valid only for low concentrations of analyte. lambert beer law at high concentrations cannot give good correlations because when the absorbance is higher. because of the substantial negative deviation to beer’s law and the lack of precision in. Why Beer Lambert Law Fails At Higher Concentrations.
From www.youtube.com
BeerLambert law in easy way YouTube Why Beer Lambert Law Fails At Higher Concentrations At higher concentrations the individual particles of analyte no longer are independent of each other. absorbance was not commonly used before approximately 1910, which is probably why planck did not directly. because of the substantial negative deviation to beer’s law and the lack of precision in measuring absorbance values above 1, it is. lambert beer law at. Why Beer Lambert Law Fails At Higher Concentrations.
From www.slideserve.com
PPT Lab 6 Saliva Practical PowerPoint Presentation ID512836 Why Beer Lambert Law Fails At Higher Concentrations beer’s law is a limiting law that is valid only for low concentrations of analyte. because of the substantial negative deviation to beer’s law and the lack of precision in measuring absorbance values above 1, it is. There are two contributions to this fundamental limitation to beer’s law. At higher concentrations the individual particles of analyte no longer. Why Beer Lambert Law Fails At Higher Concentrations.
From www.learnatnoon.com
What are some Limitations of Beer Lambert Law? Noon Academy Why Beer Lambert Law Fails At Higher Concentrations absorbance was not commonly used before approximately 1910, which is probably why planck did not directly. lambert beer law at high concentrations cannot give good correlations because when the absorbance is higher. beer’s law is a limiting law that is valid only for low concentrations of analyte. At higher concentrations the individual particles of analyte no longer. Why Beer Lambert Law Fails At Higher Concentrations.
From www.researchgate.net
1 a Schematic representation for BeerLambert law for the measurement Why Beer Lambert Law Fails At Higher Concentrations lambert beer law at high concentrations cannot give good correlations because when the absorbance is higher. absorbance was not commonly used before approximately 1910, which is probably why planck did not directly. beer’s law is a limiting law that is valid only for low concentrations of analyte. At higher concentrations the individual particles of analyte no longer. Why Beer Lambert Law Fails At Higher Concentrations.
From thechemistrynotes.com
BeerLambert Law Statement, Derivation, Applications, Limitations Why Beer Lambert Law Fails At Higher Concentrations There are two contributions to this fundamental limitation to beer’s law. because of the substantial negative deviation to beer’s law and the lack of precision in measuring absorbance values above 1, it is. beer’s law is a limiting law that is valid only for low concentrations of analyte. At higher concentrations the individual particles of analyte no longer. Why Beer Lambert Law Fails At Higher Concentrations.
From studycorgi.com
Study of Concentrations Using the BeerLambert Spectroscopic Law Free Why Beer Lambert Law Fails At Higher Concentrations absorbance was not commonly used before approximately 1910, which is probably why planck did not directly. beer’s law is a limiting law that is valid only for low concentrations of analyte. because of the substantial negative deviation to beer’s law and the lack of precision in measuring absorbance values above 1, it is. At higher concentrations the. Why Beer Lambert Law Fails At Higher Concentrations.
From www.researchgate.net
Schematics demonstrating the original BeerLambert Law and Modified Why Beer Lambert Law Fails At Higher Concentrations There are two contributions to this fundamental limitation to beer’s law. At higher concentrations the individual particles of analyte no longer are independent of each other. beer’s law is a limiting law that is valid only for low concentrations of analyte. because of the substantial negative deviation to beer’s law and the lack of precision in measuring absorbance. Why Beer Lambert Law Fails At Higher Concentrations.
From www.slideserve.com
PPT Protein Concentration Determination PowerPoint Presentation ID Why Beer Lambert Law Fails At Higher Concentrations There are two contributions to this fundamental limitation to beer’s law. At higher concentrations the individual particles of analyte no longer are independent of each other. beer’s law is a limiting law that is valid only for low concentrations of analyte. because of the substantial negative deviation to beer’s law and the lack of precision in measuring absorbance. Why Beer Lambert Law Fails At Higher Concentrations.
From www.youtube.com
Calculate concentration from UVVis absorbance using BeerLambert's law Why Beer Lambert Law Fails At Higher Concentrations absorbance was not commonly used before approximately 1910, which is probably why planck did not directly. There are two contributions to this fundamental limitation to beer’s law. because of the substantial negative deviation to beer’s law and the lack of precision in measuring absorbance values above 1, it is. lambert beer law at high concentrations cannot give. Why Beer Lambert Law Fails At Higher Concentrations.